
High-performance liquid chromatography (HPLC) is an analytical technique that separates components in a mixture to determine what substances are present. Not only can you see what is in a mixture, but you can also use HPLC systems to determine the quantities of each substance too, giving an accurate view of your sample.
In HPLC, a liquid sample is injected into a solvent stream, known as the mobile phase, which flows through a column packed with a stationary phase. A high-pressure pump controls the flow of the mobile phase, while an injector introduces the sample into this stream.
As the sample travels through the column, its components separate based on their interactions with the mobile and stationary phases. This separation process allows different substances in the mixture to be identified. A detector then captures the eluted compounds, providing essential data for further analysis.
Normal phase chromatography uses polar compounds for the stationary phase (e.g. silica particles) while the mobile phase is non-polar (e.g. hexane). In this type of chromatographic analysis, sample components are separated based on how much they interact with the polar stationary phase.
In simple terms, the higher the polarity of a substance, the slower it will move through the column. Therefore, more polar compounds will have a slower flow rate and non-polar molecules will have a quicker flow rate. This happens because, during normal phase elution, the polar compounds in the stationary phase strongly attract other polar compounds in the sample, and the non-polar compounds in the mobile phase attract the non-polar compounds in the sample mixture.
Then, using the measured flow rates, we can determine what molecules are present in the sample mixture based on what we know about the polarity and flow rate of existing substances.

In contrast to normal phase HPLC, reversed-phase HPLC has a non-polar stationary phase using modified silica particles and a polar mobile phase, typically a mixture of water and an organic solvent like methanol.
This means the movement of highly polar compounds through the column is faster than molecules with lower polarity – the opposite of in normal phase HPLC, hence the name reversed-phase HPLC.
You may be wondering why there’s a need for reversed-phase HPLC if it’s just the opposite of normal phase HPLC, but the reason is quite simple. Reversed-phase HPLC is more replicable and therefore more reliable than normal phase HPLC, both of which are fundamental parts of scientific research.
After the sample mixture has passed through the HPLC column, the separated compounds reach the detector. While there are many different detection methods, the fundamental job of the detector is to indicate how long it takes the molecules to elute and the respective concentration.
This is visualised in a graph, also known as a chromatogram.
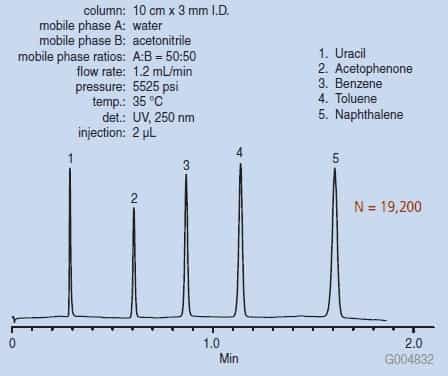
On a chromatogram, the X-axis indicates the time it took for the substance to reach the detector (also known as retention time). The Y-axis indicates the detector response (i.e. how much the molecule interacts with the detector). The exact meaning of the Y-axis will depend on the detection method used, for example, if a UV detector is used, the Y-axis will show how much ultraviolet light is absorbed by the molecule in question.
Thanks to the chromatogram, we know the retention time and detector response of each compound in the sample, which can be compared to the properties of known compounds to identify the various components in the sample.
Finally, we can work out the concentration of each substance using the area under the curve on the chromatogram – the larger the area under the curve, the higher the concentration of that substance.
We know that HPLC separation occurs based on the interaction between the compound with the stationary and mobile phases, but in a gas chromatographic analysis, separation occurs based on the volatility of each compound.
Gas chromatography still uses stationary and mobile phases like HPLC, but the mobile phase is typically an unreactive or inert gas that is unlikely to absorb other substances, like hydrogen. The stationary phase is usually tiny silica particles or another chemical that selectively attracts a certain molecule.
This means fewer volatile molecules will move slowly through the column as they interact with the stationary phase more, while more volatile molecules move faster through the column with the mobile phase.

When equipment is in full working order, it’s relatively straightforward to identify compounds using chromatography techniques. However, if your equipment is poorly maintained, you may find the results of your analysis are skewed, therefore compounds can’t be identified correctly.
LC Services stock a wide range of chromatography equipment and parts to ensure your analyses are as accurate and reliable as possible. We also offer a range of service contracts to highlight issues as soon as possible to reduce costs and bring you peace of mind that your lab is running at peak performance.
